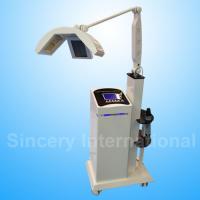
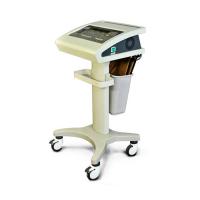
Product Specification
| Condition | New |
| Brand | MAKO |
| Properties | Medical X-Ray Equipment & Accessories |
Product Descriptions
The Infraredx Makoto Intravascular Ultrasound System brings advanced imaging
tools to doctors and nurses in a cath lab. It brings a practical and easy-to-use
approach to simplify interaction and communication during a procedure.
Infraredx. “IVUS NIRS technology has the potential to dramatically change the
field of interventional cardiology. Two unique modalities in one catheter provide
cardiologists twice the information of other imaging catheters, helping to inform
personalized treatment decisions.
The design wraps InfraRedX's advanced technology in an aesthetically pleasing
and easy-to-use form to produce a solution that brings life saving technology to
hospitals around the globe. By simplifying the details and focusing on the user,
the Makoto Intravascular Ultrasound System provides real value that benefits
patients, caregivers and InfraRedX alike.
The Makoto™ Intravascular Imaging System, with accompanying Dualpro™
IVUS NIRS Catheter, is the only imaging system on the market today that
utilizes not just intravascular ultrasound (IVUS) but also Near-Infrared
Spectroscopy (NIRS) to help clinicians visualize vessel structure and gain
valuable insights into plaque composition. Our revolutionary technology is the
only FDA-cleared imaging technology indicated for the detection of Lipid Core
Plaque (LCP).
The Makoto Intravascular Imaging System and Dualpro catheter is the only
technology on the market to identify vessel structure and plaque composition
using IVUS NIRS. Its launch follows market approval from Japan’s
Pharmaceuticals and Medical Devices Agency (PMDA) in August 2017.
Dualpro is the only imaging catheter on the market equipped with extended
bandwidth IVUS technology. By emitting and carefully processing a broad band
of frequencies, the Dualpro IVUS provides best-in-class image resolution without
compromising depth of field. Data collected from the NIRS technology are
translated into a Chemogram, an easy-to-interpret, color-coded map to identify
lipid core plaque (LCP), which can help distinguish between stable plaque and
dangerous LCP. Coupled together, IVUS NIRS arms cardiologists with
unparalleled insights into the role LCP plays in heart disease.
There are several global landmark studies, including the Lipid-Rich Plaque (LRP)
Study, currently underway, which underscore the importance of identifying LCP
for the prediction, and ultimately prevention, of serious heart attacks. Results of
the prospective, multi-center LRP Study, along with a U.S. market launch of the
Makoto Imaging System and Dualpro IVUS NIRS catheter, are anticipated in 2H
2018. The technology is currently the only FDA-cleared dual-modality catheter
and imaging system indicated for the detection of LCP.About the Makoto Intravascular Imaging System
The Makoto Intravascular Imaging System, with accompanying Dualpro™
IVUS NIRS catheter, is the only technology on the market to identify vessel
structure and plaque composition using IVUS NIRS. Combining best-in-class
image resolution with insight into the composition of plaque via Chemogram,
IVUS NIRS provides cardiologists with the tools needed to potentially predict,
and ultimately prevent serious heart attacks.
This unit has been fully tested, serviced, and refurbished at our headquarters in
Temecula, California. We also include a full warranty. Our trained technicians
have tested and serviced this equipment to ensure it is operating to the
manufacturer’s specifications.
Price is set on 22,400 USD





 Safe and secure payments using Abraa safe trade systems
Safe and secure payments using Abraa safe trade systems  \
\