مواصفات المنتج
| الماركة | DRAGON CROWN MEDICAL Co., LTD |
| الحالة | New |
مواصفات المنتج
Introduction
Kyphoplasty instruments kit was developed by our company together with experts in orthopedics and radiology field. It is used for Percutaneous Kyphoplasty surgeries to treat vertebral compression fractures(VCF) resulted from osteoporosis, vertebral benign and malignant tumors.
Indication
1. Recent thoracolumber compression fractures caused by osteoporosis and without combined nervous system injury.
2. Old thoracolumber compression fractures and intractable low back pain caused by serious kyphosis combined with fracture.
3.Secondary multi-segment compression fracture caused by adjacent osteoporosis compression fracture.
4. Pathological compression fracture caused by tumors such as vertebral lymphoma.
Contraindication
1.Osteomyelitis, epidural cyst.
2.Vertebral compression fractures with more than 70% height loss.
3.Severe spinal cord compression or secondary spinal stenosis at the level of fractured vertebral body.
4.Severe cardiopulmonary dysfunction, coagulation disorders, sepsis and the other diseases that is not suitable for the operation.
Matched Instruments
1.Puncture needle
2.Cement injection device
3.Bone drill
4.Pushing rod
5.Dilating tube
6.Working cannula
7.kyphoplasty balloon
8.Inflation device
Balloon Specification
| Part No. | Max. Diameter | Max. Length |
| LG08209 | 12mm | 19mm |
| LG08208 | 14mm | 17mm |
| LG08207 | 17mm | 22mm |
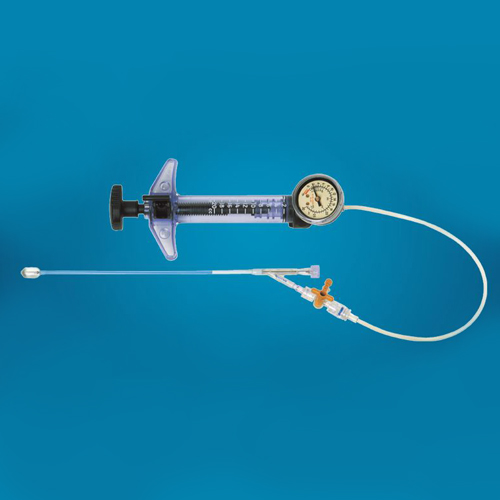
Inflation Device’s advantage
1.Interventional devices
2.Maximum 30atm, 20ml volume
3.Spiral design ensures precise pressure
4.3-Way Stopcock for option
6.Consistant pressure output
7.Sterile packaging
8.Unique ergonomical design



 طريقة دفعة آمنة وموثوقة باستخدام منصة عبرة للدفع عبر الانترنت
طريقة دفعة آمنة وموثوقة باستخدام منصة عبرة للدفع عبر الانترنت  \
\